
When it comes to the design of batteries, we want to maximize charge while minimizing volume and weight. The use of nanoparticles can help achieve these goals. However, as I was just reading in one of my favorite websites, Phys.org, it is very difficult to control the material properties when working with nanostructures.
Using Silicon as the Electrode Material in Lithium-Ion Batteries
In a lithium-ion battery, graphite is commonly used as the material for the negative electrode (during discharge). The article describes research performed by a group from Stanford University that instead uses silicon as the electrode material. Why? Silicon can hold up to ten times as much charge as graphite.
The researchers hope to produce lithium-ion batteries that contain twice the amount of energy density as today’s batteries. However, at these sizes, silicon is a much more fragile and brittle material than graphite and does not survive the charging and discharging required of such a battery.
Working with Nanoparticles in COMSOL Multiphysics®
In addition to the issues mentioned above, nanoparticles can also be difficult to model. The example below shows a typical particle that Professor Zho’s research group at Indiana University-Purdue University (IUPU), Indianapolis, wanted to model in their lithium-ion battery. They were able to create a mesh from an image of this nanoparticle and bring it into the COMSOL Multiphysics® software. What happens next? How do you define the different parts of the particle?
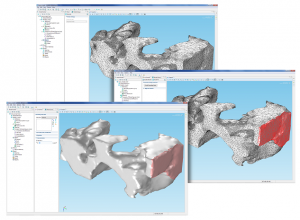
Meshed nanoparticle model, courtesy Professor Likun Zho’s research group at IUPU, Indianapolis.
COMSOL Multiphysics enables you to work with meshes and specifically select regions of elements for a group, where you can then define a domain or boundary setting. Known as Mesh Selection, this feature opens up a world of possibilities to those who work with image-to-mesh generating programs.
Next Step
Learn more about the features available in the COMSOL® software for models involving nanoparticles. Contact us to evaluate the software by clicking the button below:




Comments (0)