
The biological and chemical processes behind the development of biopharmaceuticals have an important effect on product quality. With its ability to deliver quick results at a lower cost, simulation is a valuable resource in studying and optimizing these techniques. Learn how COMSOL Multiphysics can benefit your modeling of biopharmaceutical processes.
The Relationship Between the Product and the Process
When looking at the future direction of the pharmaceutical industry, biopharmaceuticals are noted as a sector of rapid growth and interest. These products — which include vaccines, allergenics, and gene therapies — are developed with biotechnology, which uses biological processes to design or manufacture drugs or other useful products. In the development of these drugs, the end goal is to provide patients with a product of the highest possible quality.
With modeling, designers can advance the design of biopharmaceutical products and analyze how they work in vivo (i.e., investigate the product’s intended function). Researchers can study how different biomolecules, which are also manipulated by viruses and bacteria, affect a living mechanism. In our Model Gallery, you will find several examples of simulating the function of biopharmaceutical products.
Modeling can also be a valuable resource for enhancing the design of production processes for biopharmaceutical products. This is often considered the second step. After designing a biomolecule, the question then becomes how to manufacture these products on a larger scale through optimized production processes. While often different from the previous type of modeling, both of these analyses may occur in a living system (e.g., production with the use of living cells in a process). Our Model Gallery features many examples of simulating these production processes, some of which we will discuss below.
As a whole, simulation is a powerful tool for improving the design of biopharmaceuticals and the processes behind their production. In addition to faster results, this method of testing fosters innovation and offers a simplified way of improving product designs and the mechanisms used in their development.
Modeling Biopharmaceutical Processes with COMSOL Multiphysics
The COMSOL Multiphysics simulation software is a valuable tool for advancing the design and performance of biopharmaceuticals. In a recent webinar, my colleague Ahsan Munir highlighted several examples from our Model Gallery that demonstrate the use of simulation in analyzing various stages of biopharmaceutical processes.
The production stage of biopharmaceuticals often involves the use of mixers and reactors. Using COMSOL Multiphysics, you can investigate the reaction mechanisms and kinetics within these designs as well as the influence of process parameters. For instance, the Laminar Flow in a Baffled Stirred Mixer model investigates the flow within the mixer, with the opportunity to add mass transport and heat transfer in order to study the flow’s impact on the concentration and temperature fields. In the Porous Reactor with Injection Needle model, you can visualize the flow and reactions within the catalytic porous bed in a reactor, addressing the flow of fluid in porous and free regions.
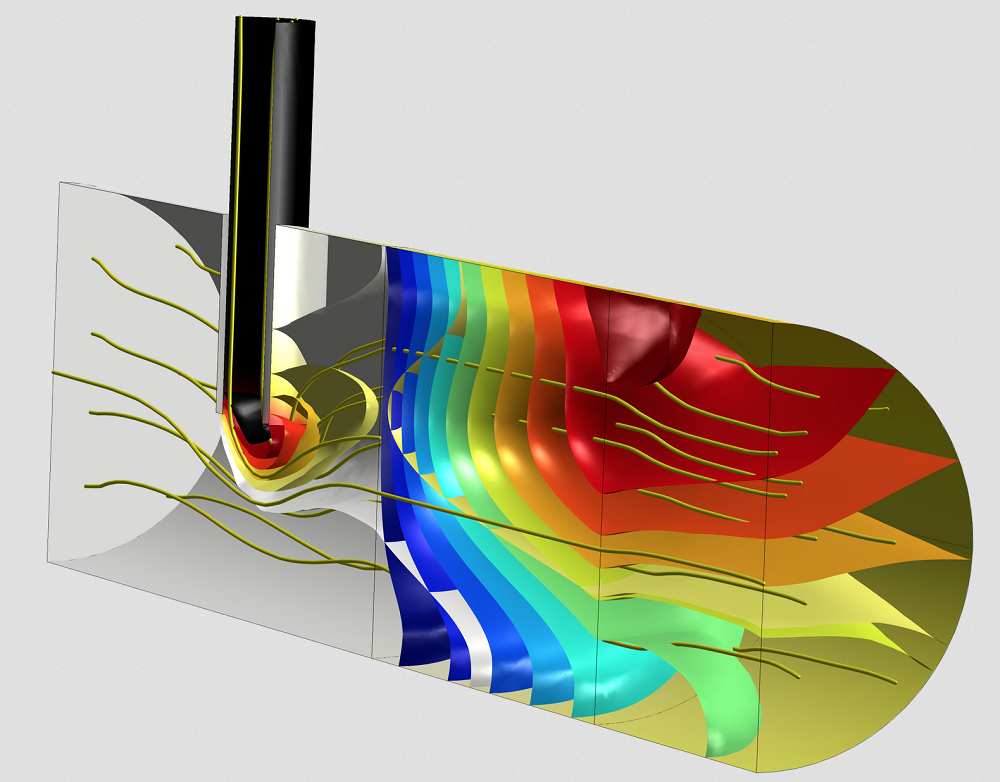
Chemical reactor model with flow streamlines and concentration isosurfaces for one of the reactants and the product species. Two species enter the reactor from different inlets and then undergo a reaction in a catalytic porous bed placed downstream from an injection needle in the reactor.
Next, we can turn to the clarification stage, which hones in on the separation of particles. Dielectrophoresis, a common method for transporting and separating different kinds of particles, is used extensively in the medical field, as biological cells possess dielectric characteristics. The Dielectrophoretic Particle Separation model shows the separation of platelets from red blood cells. This example indicates the particle trajectories both with and without the applied dielectrophoresis force as well as the electric field within the microchannel. You may also want to read my colleague Bjorn Sjodin’s blog post featuring a dielectrophoretic separation app.
This brings us to the purification stage. High-performance liquid chromatography is a technique that separates closely related chemical species within a mixture and then identifies and quantifies them. Valued for its well-controlled nature and versatility, this separation technology is often used in the development of pharmaceutical and biological products. With the Liquid Chromatography model, you can investigate the concentrations of the components in the mobile phase as well as how external heating affects the material behavior.
Last is the finishing phase. When looking to dry heat-sensitive substances like blood plasma and antibiotics, freeze-drying is a viable option. Along with helping to preserve products for a longer period of time, this technique also makes it easier to transport the products by removing water from them, thus making them lighter. COMSOL Multiphysics features A Transient Analysis of Freeze-Drying model that demonstrates a common test case for many set-ups of this technique: Ice sublimation within a vial in vacuum-chamber conditions. Using this example, you can explore how the temperature and heat changes from the beginning to the end of the drying process.
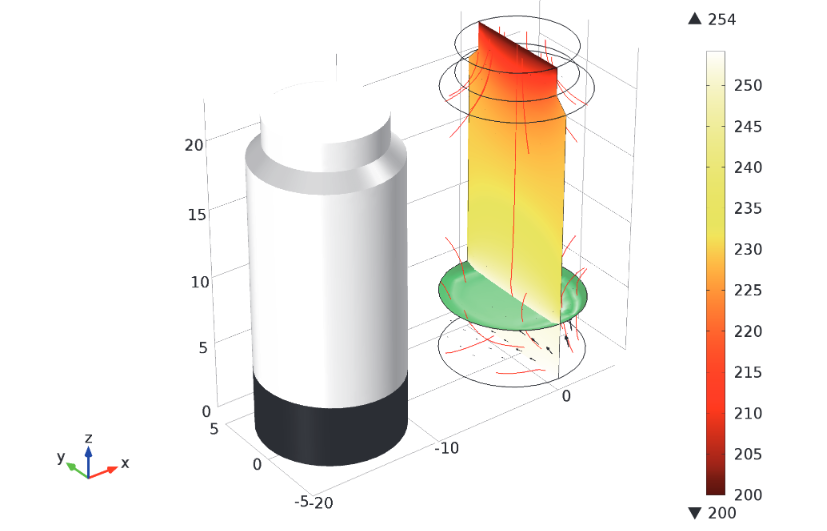
A model depicting the temperature and heat flux at the end of the drying period.
Concluding Thoughts and Next Steps
This blog post and our recent webinar provide just a brief introduction to the many benefits of modeling biopharmaceutical processes. We encourage you to further explore our Model Gallery to find additional models that can be applied to the analysis and optimization of biopharmaceutical processes. Please contact us if you would like to learn more.




Comments (0)