Electrochemical Capacitor with Porous Electrodes
Application ID: 91371
Electrochemical supercapacitors feature relatively higher energy densities than conventional capacitors. With several advantages, such as fast charging, long charge–discharge cycles, and broad operating temperature ranges, electrochemical supercapacitors have found wide applications in hybrid electric vehicles.
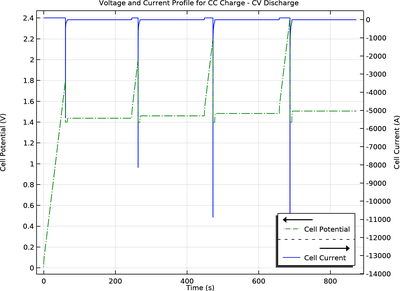
This 1D tutorial models the current distribution and electrode utilization in the porous electrodes in an electrochemical capacitor. The Nernst-Planck equations are used to model transport by diffusion and migration of the binary electrolyte, using tortuosity and porosity parameters to derive effective transport parameters for the porous electrodes.
Simulations are run using constant current-constant voltage and constant power charge/discharge loads.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Battery Design Module, Corrosion Module, Electrochemistry Module, Electrodeposition Module, or Fuel Cell & Electrolyzer Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



