Electrochemical Treatment of Tumors
Application ID: 253
This model incorporates the transport and electrolytic reaction in the treatment of tumor tissue.
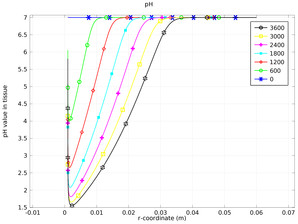
Oxygen evolution at the anode produces protons, which lowers the pH, while chlorine production also leads to lowered pH through the hydrolysis of chlorine. One effect of a low pH is the permanent destruction of haemoglobin in the tissue, resulting in the eradication of tumor tissue.
This model uses the Nernst-Planck Equations interface to predict the transport and reaction in the electrolysis of tumor tissue in a liver.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Battery Design Module, Corrosion Module, Electrochemistry Module, Electrodeposition Module, or Fuel Cell & Electrolyzer Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



