Galvanized Nail
Application ID: 14015
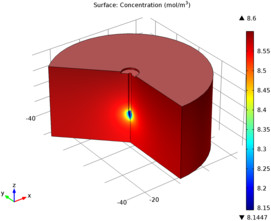
This tutorial example serves as an introduction to the Corrosion Module and models the metal oxidation and oxygen reduction current densities on the surface of a galvanized nail, surrounded by a piece of wet wood, which acts as electrolyte. The protecting zinc layer on the nail is not fully covering, so that at the tip of the nail the underlaying iron surface is exposed. First the electrolyte conductivity and the electrode reaction kinetics are modeled to obtain a secondary current distribution (concentration variations in the cell are not accounted for), in a second part the oxygen transport is included to model a tertiary current distribution.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
- COMSOL Multiphysics® and
- either the Battery Design Module, Corrosion Module, Electrochemistry Module, Electrodeposition Module, or Fuel Cell & Electrolyzer Module
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



