Water and Carbon Dioxide Co-Electrolysis in a Solid Oxide Electrolyzer Cell
Application ID: 121571
This example models co-electrolysis of H2O and CO2 using a solid oxide electrolyzer cell.
The model includes the full coupling between the mass balances and gas flow in the H2 and O2 gas diffusion electrodes, the momentum balances in the H2 and O2 gas flow channels, the energy balance across the cell, the balance of the ionic current carried by the oxide ion, and an electronic-current balance. A reversible water gas shift reaction is included in the H2 gas diffusion electrode and H2 gas flow channel.
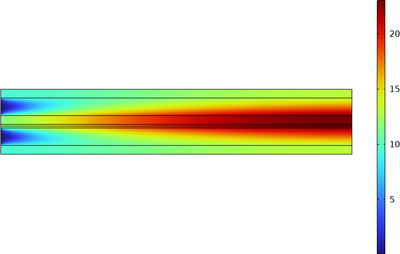
The model computes the spatial distributions of the various species across the gas diffusion electrodes and gas flow channels. The spatial distribution of the total current density along the electrode length is also evaluated in the model using the general projection operator.
This model example illustrates applications of this type that would nominally be built using the following products:
however, additional products may be required to completely define and model it. Furthermore, this example may also be defined and modeled using components from the following product combinations:
The combination of COMSOL® products required to model your application depends on several factors and may include boundary conditions, material properties, physics interfaces, and part libraries. Particular functionality may be common to several products. To determine the right combination of products for your modeling needs, review the Specification Chart and make use of a free evaluation license. The COMSOL Sales and Support teams are available for answering any questions you may have regarding this.



